I’m pretty sure your quotes are actually from the China study, not the NIAID study.
It isn’t. The NIAID study was not mostly performed on the most ill patients. Read the article yourself.
It appears you are right. Why in that article does it not talk about results vs. control for the study it is talking about? Skimming through it while working tricked me into thinking those were the numbers for this study. So Fauci said it does not improve mortality rates but does improve time to recovery for the people who were going to live anyways. Do I have that right this time?
My guess is that it makes a slight difference, but it needs to be administered early on to get most of the advantages. If I recall correctly, South Korea was using remdesivir early on in conjunction with their contact tracing.
If I’m right, this means we’ll need lots of it and lots of testing and contact tracing capacity.
The good news long-term is that remdesivir was designed to treat Ebola and Marburg virus, if they tweak it to target COVID-19 more effectively, there’s probably a decent chance they can improve its impact.
I’ve seen that like 10 times on Facebook in the last few hours
No, if people go to work and kids go to school as before there’s no way. (If you meant “avoiding large gatherings” including work and school, okay, but I’m taking the post literally as written.)
Go re-read the articles very carefully. I assume it was this one?
Here’s a bigger excerpt. The excerpt up thread was shorter, thus making it more confusing. But it’s sandwiched in between two paragraphs on the China study, so that’s what I’m 99.9% sure it’s referring to.
In the China study, also published Wednesday in The Lancet, investigators found that remdesivir “did not significantly improve the time to clinical improvement, mortality, or time to clearance of virus in patients with serious COVID-19 compared with placebo.”
There was a 23% improvement in time to clinical improvement for remdesivir compared to placebo, but the difference was not statistically significant. At the median, remdesivir-treated patients improved in 20 days compared to 23 days for placebo patients. At one month, 14% of the remdesivir patients had died compared to 13% of the placebo-treated patients.
The China study enrolled patients with more severe Covid-19 than the study conducted by NIAID. The China study was also stopped early because of difficulties enrolling patients as the pandemic waned in China.
Not quite. He said mortality “trended down” with remdesivir. That sounds like Fauci/doctor talk for some sort of inconclusive but positive result - perhaps not statistically significant, or perhaps the raw numbers don’t show it but the age adjusted numbers do.
It improves the time to recovery, though, which is really important from a broad perspective and has both positive (economic) and negative (raw death totals) effects for humanity.
Another reason the donors want everyone back to work. If everyone goes back, those who gave raises can rescind them.
The fact they haven’t released the data yet to me seems odd. It will be interesting to see. Apologies for getting the data in that article mixed up. I am working and skimming this stuff at the same time. I assumed the data would be regarding the study that was the subject of the article which was obviously a bad assumption.
Regardless of the outcome, barring some very large impact, it will be hard to square the results with that China study and know which one is right.
I did! Here’s the context after removing the paragraphs citing Bach’s criticisms:
The company’s study is enrolling approximately 6,000 participants from 152 different clinical trial sites all over the world. The data disclosed Thursday are from 397 patients, with a statistical comparison of patient improvement between the two remdesivir treatment arms — the five-day and 10-day treatment course. Improvement was measured using a seven-point numerical scale that encompasses death (at worst) and discharge from hospital (best outcome), with various degrees of supplemental oxygen and intubation in between.
The study design means that by itself it doesn’t reveal much about how well remdesivir is working, because there is no group of patients who were not treated with the drug. The conclusion is that the two durations of treatment are basically the same
[Bach quotes excised]
In the study, the median time to clinical improvement was 10 days in the five-day treatment group and 11 days in the 10-day treatment group. More than half of the patients in both groups were discharged from the hospital by day 14. At day 14, 64.5% of the patients in the five-day group and 53.8% of the patients in the 10-day group achieved clinical recovery.
Patients in the trial generally lived, though this may be because their illness was not that severe to begin with. For most of the study, patients already on ventilators were not enrolled.
Eight percent of the patients treated with five days of remdesivir died, compared to 11% of the patients treated for 10 days…
There’s no change of reference through any of these sections, so it seems to me that they must all be referring to the same study. That study being “The company study” referred to in the opening paragraph.
The article concludes by citing results from the Chinese study, which is where you get the “23% improvement in time to clinical improvement compared to placebo” from.
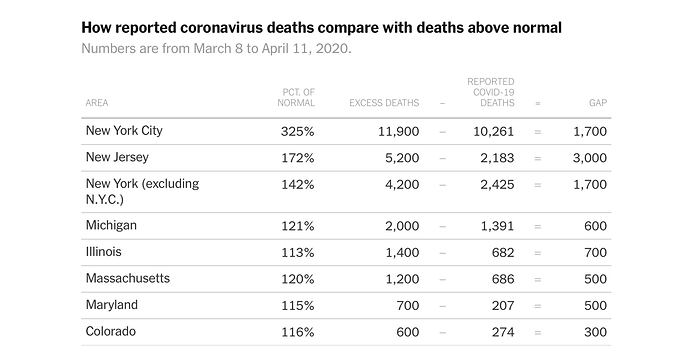
Taking those seven states, you are looking at an undercount of around 8k deaths. I suspect it is easily 10k nationwide.
Honestly I think it’s more but I am fine basing it on the overall death rate. But that means the US has had almost 70k deaths.
Ya I am a moron. I apologize for that, was definitely not trying to spread faulty info or be an ass. Anyone know when and how the data for the NIAID study gets released?
No worries, the Bach bit where it mentioned the NIAID trial made it less clear. Started doubting myself to be honest. Not sure when the proper study gets released.
South Korea now has 292 cases of people who had the virus, then tested negative and then tested positive again.
However they believe these are because tests are detecting remnants of the dead virus and not due to reinfection.
I’m also pretty sure you’re wrong about this, fwiw. Look at the numbers you posted upthread. They were mostly showing that Dems were likely being disproportionately impacted in red states. For example, you said something like 40% of the cases were in Dem counties in Indiana. But Hillary only got 37% of the vote, and slightly less than 40% of the counties are blue. Obviously the population is not evenly spread out, but I still think this is evidence of Dems being impacted unevenly.
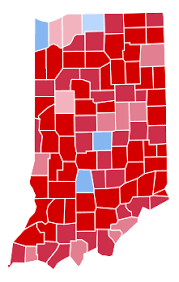
You said something like 58% of the cases in Louisiana were black? Only 32% of the population there is. I’m not going to go stat by stat, but every single one jumped out at me as not proving your point, unless I’m misunderstanding your point.
If your point is that Republicans are being impacted too, well of course they are. If your point is that it’s unpredictable, then yes of course it is. But so far, most evidence seems to show more Democrats are dying.
As a country we seem to be damn close to a net of 1 and that’s with NY clearly below 1. Cases per day seem pretty flat. It’s hard to say how much is community growth and how much is finally getting caught up on institutional cases (prisons, nursing homes).
I don’t know of a good source with some kind of “mask compliance” factor.
If I have to guess, the states rushing to more fully open are also states with poor mask wearing so we won’t even get an answer until states like PA NJ and NY open.
Seems legit from the article. I think the key message buried in there is that effectiveness is likely better early. (Similar to AIDS treatments)
Hard part is that it’s an IV drug so only going to give to hospitalized patients.
Any true positive signal at this point is worthy of further study. No problem with compassionate care use as well (note that patients already on vents were NOT part of the study).
I finally allowed an Apple News link since this is paywalled on LA Times. Such aids. Can’t even search in the page. Am I missing it or does this plan not mention restaurants and bars at all?
This seems to be actual document which offers very little in the way of details. I guess they’re still hashing out bars and restaurants.